Stopping glioblastoma stem cells from reproducing and spreading to other brain tissues is a top priority, since the cancer kills hundreds of thousands yearly. The good news is that researchers from UT Southwestern Medical Center have discovered a molecular pathway responsible for the spread of glioblastoma to surrounding tissues, and also an existing drug that was able to curb tumor growth in animal models.
“Glioblastoma’s invasive property is perhaps its most formidable barrier to treatment,” says Dr. Amyn Habib, an associate professor of neurology at the school and and a staff physician at the Dallas VA Medical Center. “We have identified a pathway that can suppress this cellular invasion, which could offer a new way to increase survival.”
Previous work done by researchers on this cancer has focused on the epidermal growth factor receptor (EGFR), which is a protein that lives on the surface of cells and promotes this type of cancer. In close to half of glioblastoma patients, the gene coding for EGFR is activated, leading to many signals being produced, subsequently driving tumor cell numbers up. The problem with previous efforts is that EGFR was inhibited in hopes of slowing the progression of the cancer, but this didn’t end up working as planned.
In this new study, the Habib lab team and others showed that when cells with greater EGFR were stimulated with ligands, the receptor acted to suppress tumors, preventing spread into healthy tissues in laboratory and animal models. More experiments showed that BIN3, a cytoskeletal protein, was responsible for the spread of the cancer.
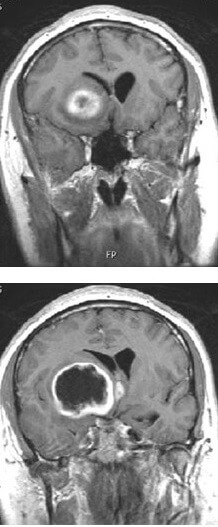
When the researchers gave animals with increased EGFR glioblastoma tumors tofacitinib, an FDA-approved arthritis drug, the tumors remained small in size and less likely to find their way to healthy brain tissue. This drug was able to work by increasing EGFR ligands and BIN3, supporting the findings of the other experiments. Furthermore, the dosed animals survived significantly longer than animals that weren’t.
“These approaches could offer new tools in our arsenal to fight glioblastoma,” concludes Dr. Habib. He also explained that tofacitinib could offer greater means to extend the lifespan of glioblastoma patients, which is only 25% and continuing to decrease with each tumor excision. As far as next steps, the team is happy to share that they will be exploring their findings more in clinical trials in September, hopefully able to translate their work to real glioblastoma patients soon.
This study is published in the journal Nature Cell Biology.







-392x250.jpg)