What petrifies you? The dark? Spiders? Clowns? A new study identifies how the brain gathers threat cues and turns them into fear. Researchers from the Salk Institute for Biological Studies have discovered a molecular pathway that filters threatening sights, sounds and smells into a singular fearful message.
The CGRP molecule allows neurons in two separate areas of the brain to gather threatening sensory cues into a unified signal. It then tags it as negative and transfers it to the amygdala, which translates the signal into fear.
Scientists hope their findings lead to new therapies for fear-related disorders, including post-traumatic stress disorder or hypersensitivity disorders like autism, migraines and fibromyalgia.
“The brain pathway we discovered works like a central alarm system,” says study senior author Sung Han, an assistant professor in Salk’s Clayton Foundation Laboratories for Peptide Biology, in a media release. “We were excited to find that the CGRP neurons are activated by negative sensory cues from all five senses — sight, sound, taste, smell and touch. Identifying new threat pathways provides insights into treating fear-related disorders.”
Prior studies revealed that different pathways independently relay sound, sight and touch threat cues to multiple brain areas. Research also showed that the amygdala, which launches behavioral responses and creates fear memories to environmental and emotional stimuli, receives heavy input from brain regions that are filled with a chemical linked with aversion, the neuropeptide CGRP. However, no one had ever found a single pathway that combined the threat cues, until now.
“Based on these two pools of research, we proposed that CGRP neurons, found especially in subregions of the thalamus and the brainstem, relay multi sensory threat information to the amygdala,” explains study co-first author Shijia Liu, a graduate student in the Han lab. “These circuits may both generate appropriate behavioral responses and help form aversive memories of threat cues.”
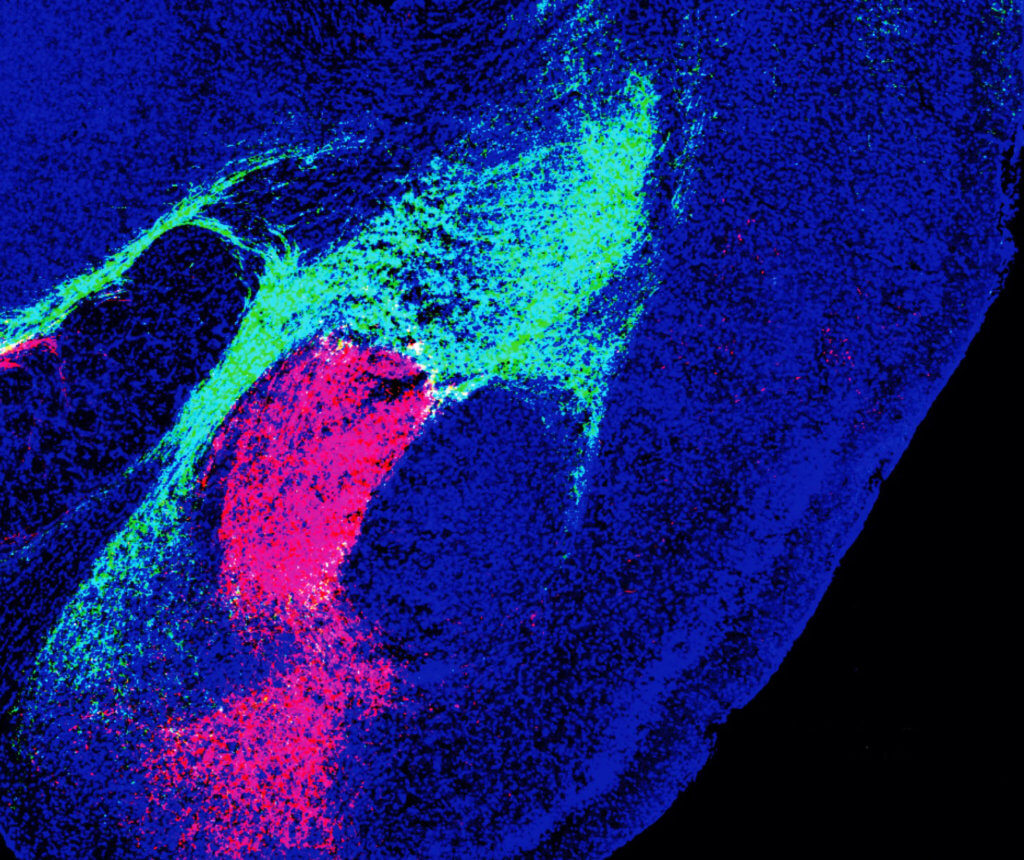
Experiments included recording CGRP neuron activity using single-cell calcium imaging while showing mice multi sensory threat cues. This allowed scientists to pinpoint which sensory modality involved which sets of neurons. They found the path signals took after leaving the thalamus and brainstem using different colored fluorescent proteins and conducted behavioral tests to measure memory and fear.
Researchers discovered that the populations of CGRP neurons in the thalamus and the brainstem projected to nonoverlapping areas of the amygdala, forming two distinct circuits. Study authors say that both populations encode threatening sights, sounds, smells, tastes and touches by communicating with local brain networks. Through their work, they found that both circuits are needed to form aversive memories.
“While mice were used in this study, the same brain regions also abundantly express CGRP in humans,” notes Han. “This suggests that the circuits reported here may also be involved in threat perception-related psychiatric disorders.”
Researchers hope to conduct future studies on how CGRP signaling in these circuits moderates disorders involving multisensory stimuli processing abnormalities, including migraines, PTSD and autism spectrum disorder.
“We haven’t tested it yet, but migraines might also activate these CGRP neurons in the thalamus and brainstem,” explains study co-first author Sukjae Joshua Kang, a postdoctoral fellow in the Han lab. “Drugs that block CGRP have been used to treat migraines, so I’m hoping that our study can be an anchor to use this kind of drug in relieving threat memories in PTSD, or sensory hypersensitivity in autism, too.”
The study is published in the journal Cell Reports.







-392x250.jpg)