Psychedelics are steadily making their way into therapists’ and doctors’ offices across the country. While the treatment is certainly unconventional and controversial, the benefits are increasingly evident. Psychedelics are used to treat a variety of syndromes, from depression to Post Traumatic Stress Disorder (PTSD). Most recently, however, researchers have sought approval for the use of psychedelics as a treatment for amyotrophic lateral sclerosis (ALS).
ALS, also known as Lou Gherig’s disease, is a nervous system disease that weakens muscles and impacts physical functions. In this disease, nerve cells break down, which reduces the functionality of muscles. As ALS progresses, symptoms become more widespread, and some muscles become paralyzed while others are weakened or unaffected. In late-stage ALS, most voluntary muscles are paralyzed.
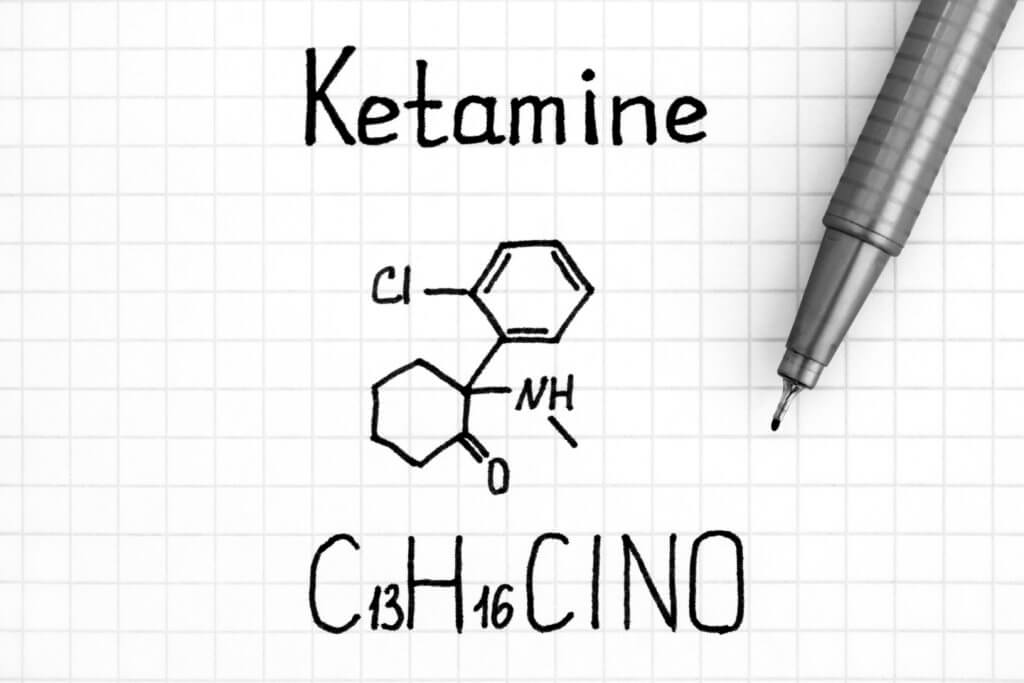
Ketamine as a potential ALS treatment
Dr. Richard Barohn is a neurologist and the executive vice chancellor for health affairs at University of Missouri Health Care, and the chief investigator of the latest study searching for a possible treatment for ALS. Barohn and his team of researchers recently received approval from the Food and Drug Administration (FDA) to proceed to Phase 2 of using psychedelic ketamine from PharmaTher, a Ketamine Pharmaceutical Company, to treat the excruciating and muscle-deterioration symptoms of ALS.
Led by Barohn, the research team at the University of Missouri will evaluate the safety and efficacy of ketamine in 36 ALS patients. In addition to testing toxicity, the goal is to examine the medicine’s impact on the disease’s severity and progression.
“We are very pleased to have supported Dr. Barohn and his team by providing information for the IND that achieved FDA acceptance to conduct the Phase 2 clinical study,” says Fabio Chianelli, CEO of PharmaTher, in a statement.
Ketamine is a dissociative anesthetic that has some hallucinogenic effects. It distorts perceptions of sight and sound and makes the user feel disconnected and not in control. One effect of ketamine is the indirect blockade of NMDA receptors, which prevents glutamate from binding and could potentially protect nerve cells from excitotoxicity. This means that on top of relieving the pain of ALS patients, ketamine could also prevent the further deterioration of muscle nerve cells. Preclinical research conducted by scientists at the University of Kansas showed that ketamine was neuroprotective, preserved muscle function, and extended life expectancy in a mouse model of ALS, supporting its development as a potential ALS therapy. Phase 2 will allow researchers to evaluate the use of ketamine in ALS patients.
Promising future
If results from this Phase 2 look promising, PharmaTher will move forward with planning a Phase 3 trial together with the FDA. President Biden last month signed into law the “Accelerating Access to Critical Therapies for ALS Act”, which will fund research for ALS and other neurodegenerative diseases. The law supports investigational therapy studies such as this one.
“ALS is a devastating neurodegenerative disease with limited treatment options and ketamine, based on preclinical research, has the potential to have a positive impact on ALS patients,” Chianelli says.
Ketamine also is approved as a therapy for treatment-resistant depression. PharmaTher is involved in an ongoing clinical trial evaluating the compound for Parkinson’s disease.
PharmaTher announced the promising news on January 18.
This research is published by ALS News Today.
Article written by Rhonda Errabelli