Imagine being able to study the intricate workings of the human brain without the need for invasive procedures. Enter brain organoids — tiny, three-dimensional structures grown from human stem cells that mimic the complexity of the brain. These innovative tools are now being used by scientists to unravel the mysteries of traumatic brain injury (TBI) and its long-term consequences for millions of people worldwide.
In a new study published in the journal Cell Stem Cell, a team of researchers led by Jesse Lai and Joshua Berlind from the University of Southern California have developed a novel method to inflict precise mechanical injuries on human brain organoids using high-intensity focused ultrasound (HIFU). This approach allows them to closely mimic the effects of TBI in a controlled laboratory setting.
“There’s really nothing out there that can prevent the injury or trauma to the brain that cause nerve cell damage,” says Ichida in a media release. “In more acute stages, patients can have difficulty concentrating and have extreme sensitivity to light and noise. Long term, there is a strong correlation between traumatic brain injury and neurodegenerative diseases, which can ultimately be fatal.”
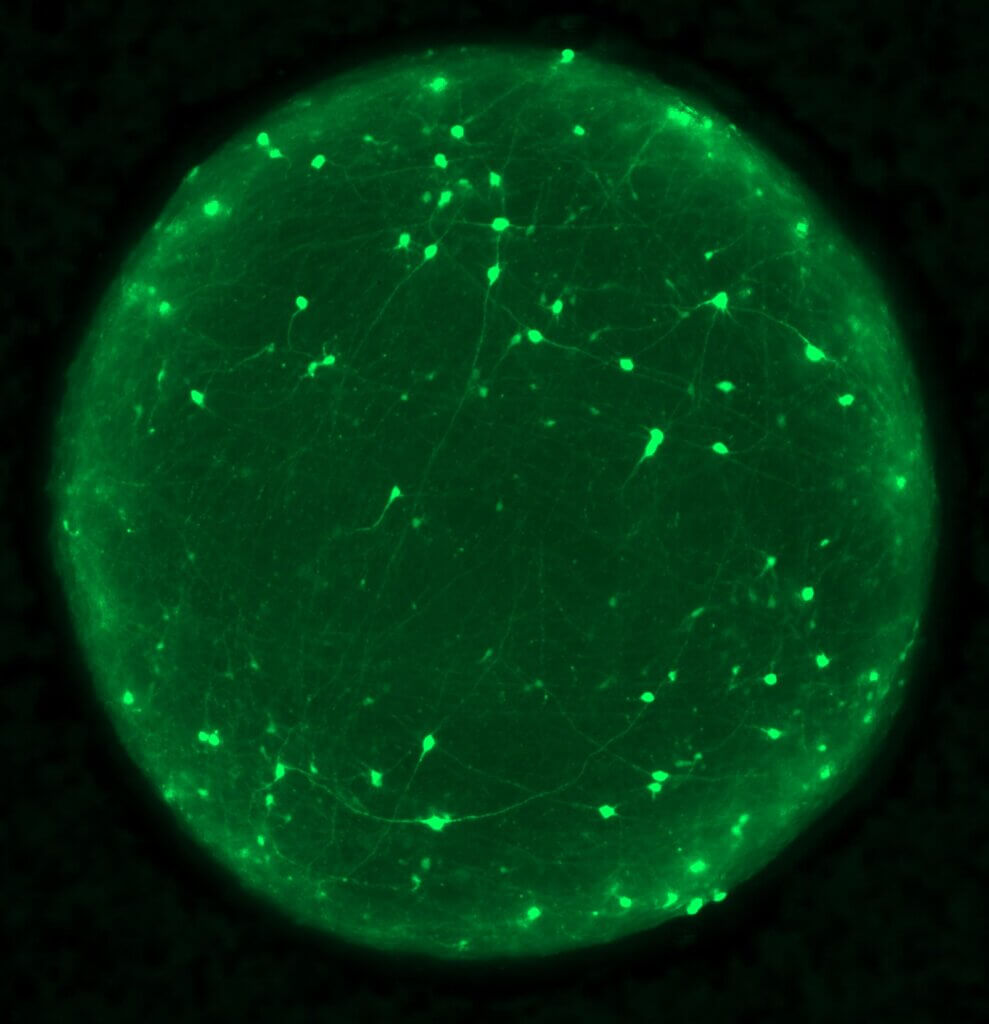
Researchers found that mechanically injured organoids displayed classic hallmarks of TBI, including neuronal death, tau protein phosphorylation, and mislocalization of the protein TDP-43. They were surprised when they observed that deep-layer neurons were particularly vulnerable to injury compared to upper-layer neurons in the organoids.

TDP-43, a protein known for its role in neurodegenerative diseases such as amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD), emerged as a key player in driving neuronal death after mechanical injury. Researchers found that injured organoids exhibited loss of TDP-43 function, leading to the incorporation of cryptic exons in its target genes, such as stathmin-2 (STMN2).
To identify potential therapeutic targets, the team performed a genome-wide CRISPR interference screen on the injured organoids. They discovered that inhibiting a mechanosensory ion channel called KCNJ2 significantly reduced neuronal death and TDP-43 pathology following mechanical injury. These findings were validated in a mouse model of TBI, where knockdown of the mouse equivalent of KCNJ2 (Kcnj2) reduced TDP-43 pathology, cell death, and even improved motor function post-injury.
Researchers also explored the interaction between TBI and genetic predisposition to neurodegenerative diseases. They found that brain organoids derived from ALS/FTD patients carrying a mutation in the C9ORF72 gene displayed exacerbated TDP-43 pathology following mechanical injury compared to healthy control organoids. Remarkably, inhibiting KCNJ2 in these patient-derived organoids mitigated the enhanced TDP-43 dysfunction and neuronal death, highlighting the potential therapeutic value of targeting this ion channel.
This study represents a significant breakthrough in our understanding of the complex mechanisms underlying TBI and its long-term consequences. By leveraging the power of human brain organoids and cutting-edge genetic tools, the researchers have identified a novel therapeutic target, KCNJ2, which could potentially mitigate the devastating effects of TBI.
The findings also shed light on the intricate relationship between TBI and neurodegenerative diseases, suggesting that mechanical injury may lower the threshold for developing conditions like ALS and FTD in genetically predisposed individuals. This highlights the importance of understanding the interplay between environmental factors and genetic risk in the development of these debilitating disorders.
“Our study suggests that one of the more effective ways of preventing the devastating effects of traumatic brain injury might be to fix TDP-43 and prevent its mislocalization early after injury,” notes Ichida.
As we look to the future, this study opens up exciting new avenues for further research and therapeutic development. The ability to model TBI in human brain organoids provides a powerful platform for screening potential drugs and interventions, bringing us one step closer to developing effective treatments for this devastating condition.