NeuroPace, a commercial-stage medical device company which specializes in epilepsy treatment, has been awarded the National Institutes of Health grant by the Brain Research through Advancing Innovative Neurotechnologies® (BRAIN) Initiative. The NIH grant will fund $9.3 million over a five-year study to demonstrate the RNS® System created by NeuroPace as a treatment for LGS.
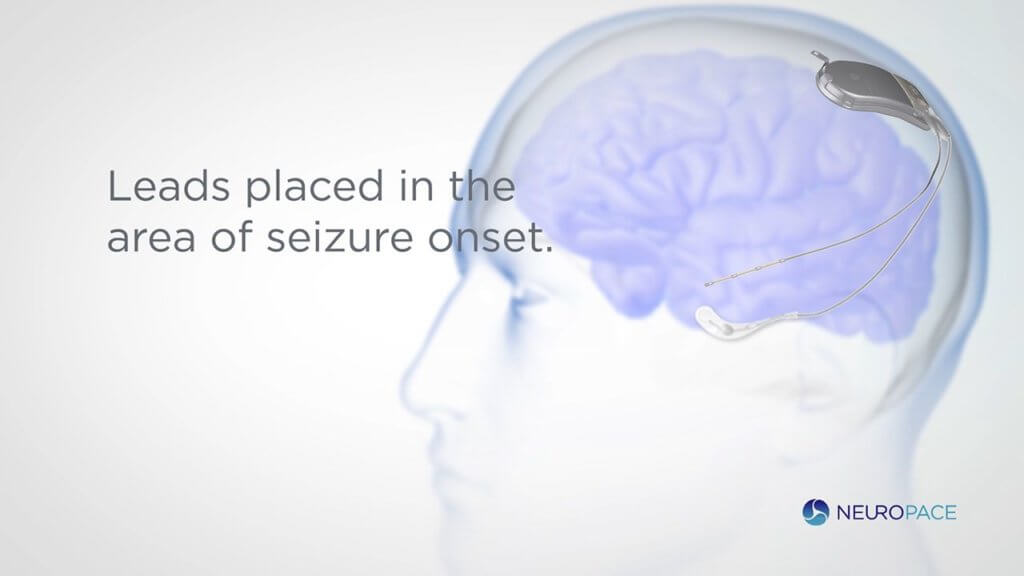
The RNS® System is a novel therapy for those suffering from drug-resistant focal epilepsy. It is the sole neuromodulation system that is brain-responsive that has been approved by the FDA. The proprietary technology continuously monitors brain activity which allows for a personalized treatment based on collected data to target seizures at their source.
The device identifies unique patterns in brain activity and delivers real-time stimulation responses that prevent the seizure from ever occurring. This record of data gives physicians visibility into their patients’ unique patterns and enables remote monitoring to optimize patient care. The RNS Systems is currently available in the United States to patients 18 and older with drug-resistant focal epilepsy and is widely covered by health insurance.
The grant from BRAIN will fund the study of the RNS System as a treatment for Lennox-Gastaut Syndrome (LGS,) which is a type of epilepsy that develops in children. LGS causes cognitive impairment and frequent seizures causing injury to patients. The Investigational Device Exemption study will evaluate the device in patients with LGS.
NeuroPace’s brain-responsive neuromodulation technology will be explored as an effective therapy for those living with LGS as well as collect crucial insights for medical professionals with the RNS System’s continuous monitoring capabilities. The comprehensive record of brain activity from patients involved in the study could lead to a better understanding of the condition.
Tracy Dixon-Salazer, Ph.D., Executive Director of the LGS Foundation and mother of an adult child living with LGS, shared, “Most people living with LGS have tried more than a dozen treatments and yet seizures persist and families live life waiting for the next seizure crisis. I am so encouraged by the research being done with the RNS System and am hopeful that this treatment can help LGS families who are in desperate need of better therapies.”
The study will include the efforts of 6 clinical study sights and 2 academic sites which will work on patient brain mapping to illustrate seizure networks. This will allow for unprecedented personalization of treatment for each participant. 20 individuals with LGS will be enrolled in the IDE study and, after FDA approval, the team will design a more comprehensive clinical study.
“We are pleased that the NIH recognizes the promise of responsive neuromodulation to potentially address the current gap in therapeutic options for patients with debilitating seizure disorders such as LGS,” said Martha Morrell, M.D., Chief Medical Officer of NeuroPace and principal investigator of the study. “These recordings obtained directly from the brain in a natural setting will show us what is happening when an LGS seizure starts and spreads. We believe that this unprecedented window to the brain will provide us with a deeper understanding of generalized onset seizure networks and help identify biomarkers in the brain that signal when a seizure is about to occur. We’re hopeful that treatment with the RNS System can ultimately improve the lives of patients with LGS and their families, and that what is learned from the brain data can also be applied to treatment of other types of generalized onset seizures.”
The goal of the study is to collect clinical and electrophysical data that can identify biomarkers and build foundational understandings to support future epilepsy research and treatment.