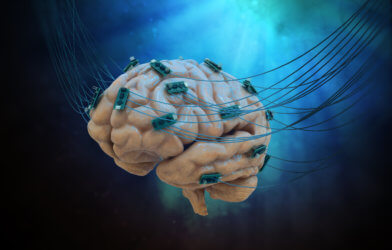
Researchers at Brown University have developed a new type of brain-computer interface (BCI) device that could eventually lead to improved therapies for patients with brain or spinal injuries. BCIs work with implantable sensors that record the brain’s electrical signals. Those signals then operate external devices like computers or robotic prosthetics, enabling disabled patients to move or communicate.
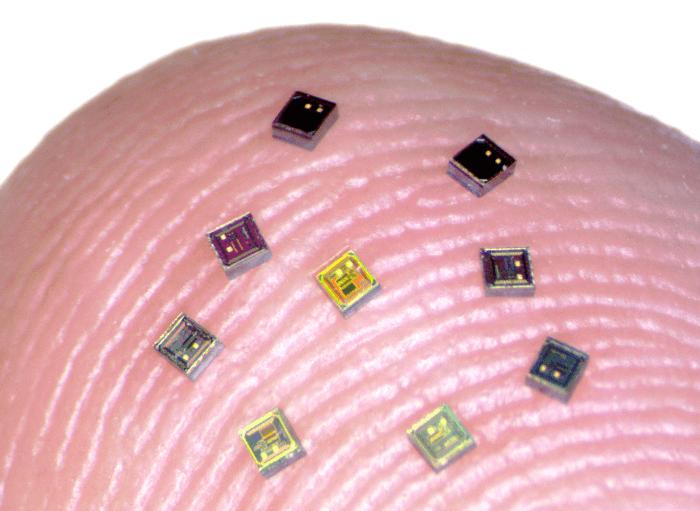
Until now, BCI systems consisted of one or two sensors processing as much as a few hundred neurons. The new device will lead to an improved system that employs grain-sized, independent, wireless microscale neural sensors called “neurograins.” Forming a wireless network, each sensor records the brain’s electrical signals and sends them to the device’s center where they are coordinated and processed.
To test the new device, the researchers implanted 48 neurograins in the outer layer of a rodent’s brain. They tested the device’s ability to record from the brain and to stimulate it. The researchers hope that stimulating the brain with the device could enable scientists to restore lost brain functions in the future. “One of the big challenges in the field of brain-computer interfaces is engineering ways of probing as many points in the brain as possible,” says the study’s senior author Arto Nurmikko, a professor in Brown’s School of Engineering, in a statement. “Up to now, most BCIs have been monolithic devices — a bit like little beds of needles. Our team’s idea was to break up that monolith into tiny sensors that could be distributed across the cerebral cortex. That’s what we’ve been able to demonstrate here.”
When developing the device, the researchers first had to figure out how to fit a complex electronic system into the grain-sized chips. Then they worked on designing the “communications hub” outside the body that’s processing the brain’s signals. It is a thin, thumbprint-sized patch that attaches to the skin of the skull. In addition to gathering the neurgrain’s data, the patch also powers them wirelessly. “This work was a true multidisciplinary challenge,” said study lead author Jihun Lee, a postdoctoral researcher at Brown. “We had to bring together expertise in electromagnetics, radio frequency communication, circuit design, fabrication, and neuroscience to design and operate the neurograin system.”
“It was a challenging endeavor, as the system demands simultaneous wireless power transfer and networking at the megabit-per-second rate, and this has to be accomplished under extremely tight silicon area and power constraints,” said Vincent Leung, an associate professor in the Department of Electrical and Computer Engineering at Baylor University. “Our team pushed the envelope for distributed neural implants.”
While the number of neurograins tested was limited due to the size of the rodent’s brain, the researchers gather based on their data that the device can support up to 770. The team hopes to increase this number up into the thousands for unprecedented insight into brain activity. “Our hope is that we can ultimately develop a system that provides new scientific insights into the brain and new therapies that can help people affected by devastating injuries,” Professor Nurmikko said.
The study was published in Nature Electronics.