Glioblastoma (GBM), the most common and aggressive form of brain cancer in adults, has long been a formidable foe for researchers and clinicians. With a median survival rate of just 12-18 months after diagnosis, and even less for recurrent cases, GBM has proven resistant to traditional treatments like surgery, radiation, and chemotherapy. However, a new study led by researchers from the Perelman School of Medicine at the University of Pennsylvania and Penn Medicine’s Abramson Cancer Center offers a glimmer of hope in the form of a novel dual-target CAR T cell therapy.
The Power of Two: Targeting EGFR and IL13Rα2
The study, published in Nature Medicine, reports on the first six patients treated in an ongoing Phase I clinical trial using CAR T cells engineered to target two proteins commonly found in brain tumors: epidermal growth factor receptor (EGFR) and interleukin-13 receptor alpha 2 (IL13Rα2). This marks the first time a dual-target CAR T cell therapy has been used in patients with glioblastoma.
“This is the first time CAR T cell therapy with two targets, rather than just one, has been administered to patients with glioblastoma,” said Dr. Stephen Bagley, an assistant professor of Hematology-Oncology, and Neurosurgery, and principal investigator in the clinical trial, in a statement. “Our results suggest that this is a step in the right direction, and this method, when delivered through a patient’s spinal fluid, could be the key to developing therapies that outsmart the complicated defense systems of GBM.”
The Promise of CAR T Cell Therapy
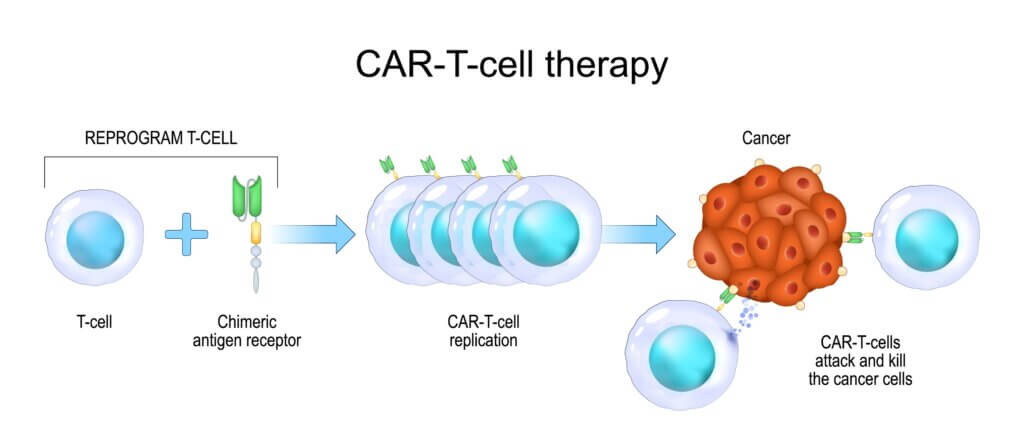
CAR T cell therapy has already shown remarkable success in treating certain blood cancers, such as leukemia. The therapy involves harvesting a patient’s own T cells (a type of white blood cell), genetically modifying them to recognize and attack cancer cells, and then reinfusing them back into the patient. However, solid tumors like GBM have proven more challenging due to their heterogeneity (not all cells within the tumor are the same) and their ability to evade the immune system.
The dual-target approach used in this study aims to overcome these obstacles by targeting two proteins that are commonly expressed in GBM tumors. EGFR is estimated to be present in 60% of all GBMs, while IL13Rα2 is expressed in over 75% of cases. By targeting both, the researchers hope to increase the chances of the CAR T cells successfully recognizing and attacking the tumor cells.
Encouraging Early Results
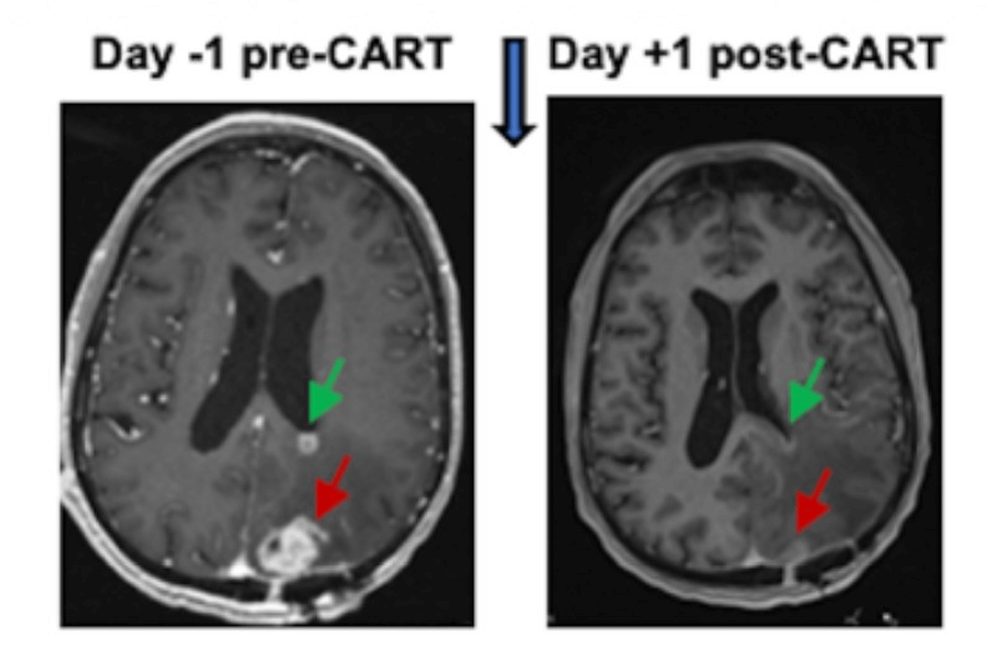
The initial results from the first six patients treated in the trial are promising. MRI scans taken 24 to 48 hours after the dual-target CAR T cells were administered showed reduced tumor sizes in all six patients, with these reductions being sustained for several months in some cases.
“We are energized by these results, and are eager to continue our trial, which will give us a better understanding of how this dual-target CAR T cell therapy affects a wider range of individuals with recurrent GBM,” said Dr. Donald M. O’Rourke, MD, a professor in neurosurgery and director of the Glioblastoma Translational Center of Excellence at the Abramson Cancer Center, and scientific advisor to the trial. “This cancer is unique in each individual, so a wider range of patients will help us determine the optimal dose, better understand effects like neurotoxicity, and more firmly establish efficacy.”
Navigating Neurotoxicity
One of the major concerns with CAR T cell therapy, especially when delivered directly to the brain, is the risk of neurotoxicity. This occurs when a toxic substance alters the activity of the nervous system, potentially disrupting or killing brain cells (neurons). In this trial, all six patients experienced substantial but manageable neurotoxicity.
Managing these side effects will be a key focus as the trial continues and the therapy is refined. The researchers hope that by carefully monitoring patients and adjusting dosages as needed, they can maximize the therapeutic benefit while minimizing the risk of neurotoxicity.
A Glimmer of Hope
While these early results are encouraging, it’s important to remember that this is just the beginning of a long journey. The ongoing Phase I trial will continue to enroll patients and gather data on the safety and efficacy of this dual-target CAR T cell therapy. If successful, it will need to progress through additional clinical trial phases before it can be considered for widespread use.
However, for patients and families facing the daunting diagnosis of glioblastoma, any glimmer of hope is welcome news. The median survival rate for recurrent GBM is less than one year, and despite decades of research, there is still no known cure. If this dual-target CAR T cell therapy can improve outcomes, even incrementally, it could make a meaningful difference in the lives of those affected by this devastating disease.
As Dr. Bagley notes, “Our challenge is getting our treatment around the tumor’s defenses so we can kill it.” With this novel dual-target approach, researchers are one step closer to overcoming those defenses and giving patients with glioblastoma a fighting chance. While there is still much work to be done, this study represents an important milestone in the ongoing battle against one of the most formidable foes in oncology.
For more information on glioblastoma research and clinical trials at Penn Medicine, visit: www.pennmedicine.org/brain-tumor.