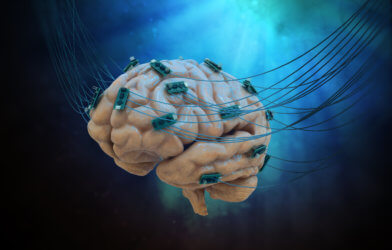
In a remarkable breakthrough, researchers from Rice University have developed a miniature, battery-free brain stimulator that could revolutionize the field of neuromodulation. This innovative device, known as the Digitally programmable Over-brain Therapeutic (DOT), is only 9 millimeters wide yet packs enough wireless power to stimulate brain activity through the dura, the tough outer layer of the brain.
Neuromodulation, the process of electrically stimulating the central or peripheral nervous system, has shown great promise in treating various psychiatric, movement, and pain disorders, as well as in restoring movement after spinal cord injury. However, the adoption of bioelectronic therapies like deep brain stimulation (DBS) has been limited by patient perception of risk, high procedural costs, and long wait times for complex procedures.
The DOT addresses these challenges by providing a minimally invasive alternative to traditional neuromodulation devices. By placing the device in the epidural space, just above the dura, researchers can activate neural tissue without direct contact, simplifying the delivery process and reducing the risk of damaging the target tissue. The incredible findings are published in the journal Science Advances.
“In this paper we show that our device, the size of a pea, can activate the motor cortex, which results in the patient moving their hand,” says study author Jacob Robinson, a professor of electrical and computer engineering and of bioengineering at Rice, in a media release. “In the future, we can place the implant above other parts of the brain, like the prefrontal cortex, where we expect to improve executive functioning in people with depression or other disorders.”
One of the most impressive features of the DOT is its ability to receive enough wireless power to generate the high stimulation currents needed for epidural brain stimulation. This is made possible by the device’s magnetoelectric (ME) antennas, which efficiently harvest energy from an external magnetic field. The DOT also boasts digitally programmable stimulation output and centimeter-scale alignment tolerances, ensuring precise and reliable neuromodulation.
To put the DOT to the test, Rice researchers conducted a series of experiments in human patients and a porcine model. During intraoperative testing, the device successfully stimulated a motor response in a human patient, triggering a hand contraction when placed directly on the motor cortex. In a second study, the DOT elicited visible thumb movements when placed above the dura, demonstrating its ability to activate brain tissue through this protective layer.
“Neurostimulation is key to enabling therapies in the mental health space where drug side effects and a lack of efficacy leave many people without adequate treatment options,” explains Robinson.
Researchers also demonstrated the DOT’s long-term effectiveness by implanting the device in freely behaving pigs for up to 35 days. Throughout the chronic experiment, the DOT consistently drove motor stimulation, showcasing its reliability and durability. Importantly, the implantation surgery took only 30 minutes and involved no contact with the brain, highlighting the simplicity and safety of the procedure.
To further assess the safety of the DOT, researchers applied an intermittent theta burst stimulation (iTBS) paradigm, which is commonly used in transcranial magnetic stimulation (TMS) therapies for psychiatric conditions. Even after delivering a total of 300 minutes of iTBS-like stimulation, researchers found no serious pathology in the stimulated brain areas, suggesting that the DOT could provide a safe analog to TMS therapies without the need for frequent clinic visits.
The potential applications of the DOT are vast, ranging from treating psychiatric disorders to restoring movement in patients with spinal cord injuries. By enabling precise neuromodulation with a minimally invasive, wireless device, the DOT could dramatically improve patient access to life-changing therapies while reducing the risks and costs associated with traditional neuromodulation procedures.
Robinson hopes these devices could ultimately be used at home, under the physician’s discretion.
“Back home, the patient would put on their hat or wearable to power and communicate with the implant, push ‘go’ on their iPhone or their smartwatch and then the electrical stimulation from that implant would activate a neuronal network inside the brain,” says Robinson.
As the field of bioelectronics continues to evolve, the development of miniature, battery-free devices like the DOT represents a significant step forward. With further refinements, such as the integration of neural sensing capabilities for closed-loop control, these innovative devices could unlock new paradigms for clinical therapy, offering hope to countless patients suffering from debilitating neurological conditions.