An unprecedented report has uncovered new details of the brain’s first line of defense. International researchers have been able to detail the development and genetic profile of a set of cells that construct the brain’s immune system.
The results of this study could better help treat patients suffering from Alzheimer’s disease and multiple sclerosis.
“Many people are familiar with how neurons connect together to send signals across the brain, but there are also blood vessels that supply the brain with oxygen, and glial cells that act as the brain’s support network and immune system,” says Takahiro Masuda, study lead author from Kyushu University’s Faculty of Pharmaceutical Sciences, in a statement. “In fact, even the most generous estimates suggest only about half of the cells in our brains are neurons, so studying the other cells is just as vital for uncovering how the brain works.”
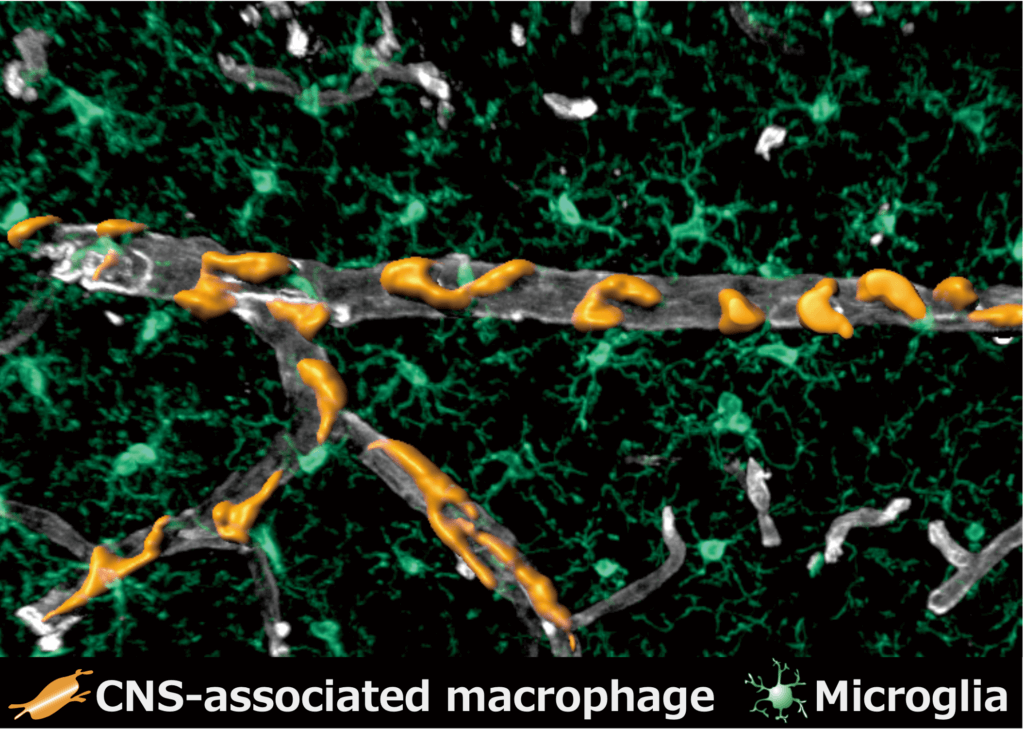
Researchers focused on a series of cells called central nervous system associated macrophages, which are a type of immune cells that protect the brain from infection. It is believed these macrophages play a part in nearly all known neurodegenerative diseases.
The team was intrigued in the macrophages surrounding blood vessels and those found in the meninges — the layers that surround the brain — known as perivascular macrophages and meningeal macrophages.
“Until now, these cells were not distinguished from other immune cells, and how and where these critical cells develop was significantly understudies,” explains Masada. “So, we investigated fundamental characteristics of these cells, such as how to distinguish them from other cells in the brain, their exact locations, how they develop, what kind of genes they express, and how they interact with other cells.”
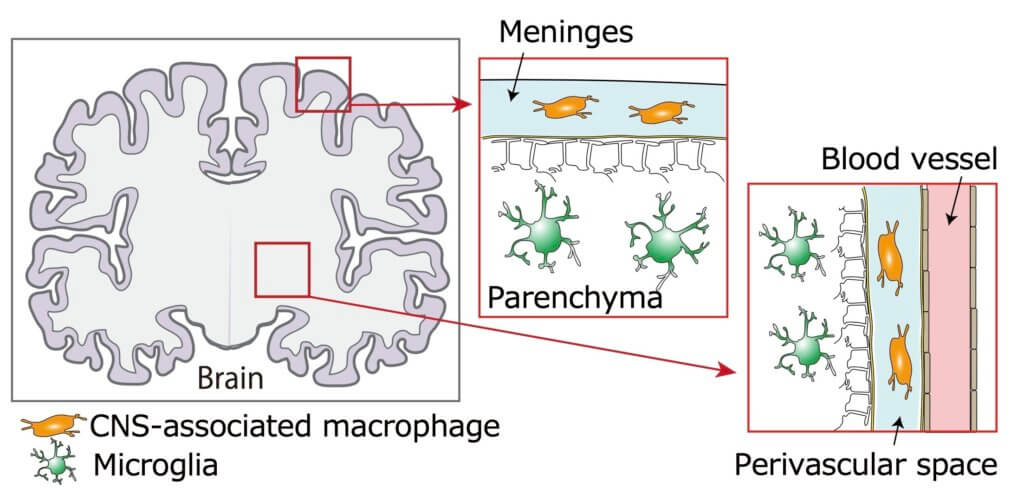
These macrophages form in the yolk sac, which is located outside the embryo. Cells migrate from the yolk sac into the brain when the organism develops. “Using a technique called ‘fate-mapping,’ the team precisely traced where these cells ended up and discerned what leads them to become perivascular macrophages and meningeal macrophages,” the researchers explain.
“We found that meningeal macrophages develop in the same way as other microglia and are formed during gestation. Perivascular macrophages, on the other hand, actually begin to form after birth, and originate from the meningeal macrophages. This was very unexpected,” says Masada.
Researchers were also able to identify the specific genes that lead to the generation of these macrophages.
“Identification of these genes will finally allow us to distinguish meningeal and perivascular macrophages from other microglia,” says Masada. “Now that we can study them individually, we can get a clearer picture of their functions.”
The study is published the journal Nature.